I took the firing to cone 9, but with a fairly long soak
that started when cone 8 just began to move, with the
temperature held almost constant until cone 9 slumped.
Cone 9 is a bit on the hot side for tortoiseshell, so the
base glaze is darker and both the base and the spots are
more transparent than they would probably be if I’d fired
them lower. (Just for reference: under common conditions,
cone 8 roughly approximates to 1240° celsius, and cone 9
to 1260°. They don’t really measure temperature, though,
and in this case I was probably holding the kiln at 1225
or so for two and a half hours.)
As I say, the photo doesn’t really show you what’s
going on here. This is really a glaze that has to be
seen "up close and personal".
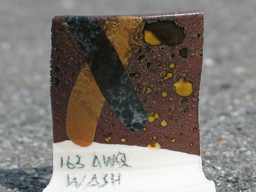
"163 AWQ" is the same wash I used on the Tortoiseshell Tenmoku test tile, shown above. It contains a lot of calcium, as one might expect from analyses of Jizhou Tortoiseshell. The brown stripe is a wash I often use on reduction Tenmoku glazes; it’s about equal parts (by volume) of Ceramic Rutile and Gerstley Borate. The gray wash is something I’m just beginning to test; it contains 8 parts Red Iron Oxide, 1 part Manganese Carbonate, and 1 part Kaolin. I’m not yet sure whether it’s a keeper. (I’ll talk more about "163 AWQ" when I’ve written the article I promised Gerry Williams at the NCECA conference, back in March.) This clay is just melting at cone 9, but is easily turned into a glaze by the addition of a little flux, as you can see from the clear spots.
I fired another test of this stuff, at cone 10 in light
reduction, and it’s smoother and shinier, but still
opaque where it’s just plain. I need to get my hands on
some of the redder material and try that. I expect that
a little wood ash will turn it into a fine Tenmoku.
You can see that it "slid" just enough to thicken
slightly at the base. I think that will prove to
be bearable; but I also think it may move a bit
less when I decrease the amount of iron oxide in it
to lighten the color. I should probably mention the
fact that I’ve been working on amber largely at the
behest of Dick Roepke, who works mainly in stoneware,
at Columbia Arts Center, which is (no real surprise)
located in Columbia, MD.
Here it is again, with a blacklight shining on it:
Now, that’s in broad daylight. (You’ll notice, if you compare the first of those two photos with the first of the next two, that there is perceptible blue color just from the UV coming out of the sky and causing the porcelain to fluoresce. Most of the blue is just the reflection of the sky, but the edge closest to the camera is reflecting clouds, and it’s still blue.)
Here it is again, this time in reduced light, without and with the blacklight:
To show you how translucent this stuff is, here’s the saucer with the blacklight behind it -- the glow you see (except at the left and right edges, where the lamp is shining on the outside) is coming through the porcelain...
I’m writing an article about this work, so I’m not going to go into details yet. (I intend to lay it out in full detail in the article.)