A friend very kindly sent me small samples of several BASF dyes. Here is one of them, Lumogen F Orange 240, in acetone. Orange 240 fluoresces extremely well, but does not absorb well at 337 nm, so the pump beam penetrates much too far into the solution. The dye is lasing, but not strongly.
Here is 7-Diethylamino-4-Methyl-Coumarin, also dissolved in acetone, pumped by the nitrogen laser. 7-DE-4-MC absorbs very well at 337 nm, as you can see by the fact that the pump beam barely gets into the solution at all.
It also happens to be a very good blue laser, though it was discovered very early. (One of its other names is “Coumarin 1”.)
Here is what happens when I add small amounts of Lumogen F Orange 240 to the solution of 7-Diethylamino-4-Methyl-Coumarin. (I got the spectra by holding an inexpensive plastic replica diffraction grating in front of the camera when I took the photos. I added the thin vertical white line, toward the right, so I could position the images correctly when I put them together for this comparison.)
The top spectrum is just 7DE4MC. It lases brightly enough that you can see the second-order spectrum at the left of the image.
Starting with the second spectrum, there is enough Orange 240 present that it is lasing.
By the fourth spectrum, there is so much Orange 240 present that the 7DE4MC can barely lase at all. It is still absorbing the pump light, however, and the extinction depth is still very shallow:
(Sorry the larger image is so small. I didn’t have the camera zoomed in enough.)
There are two significant things going on here. First, notice that as the concentration of Orange 240 gets higher, the wavelength at which it lases gets longer, up to a point. This is because the absorption curve slightly overlaps the emission curve, and when the concentration is high the dye absorbs enough of the short-wavelength end of its emission spectrum that it can only lase at longer wavelengths. This is true of all or nearly all dyes, and is one way of tuning the output of a dye laser, though it is (obviously) not very precise.
Second, notice that as the concentration of Orange 240 gets higher, the position of the blue laser emission moves very slightly to shorter wavelengths. Let’s think about this for a moment. While it is true that adding orange dye dilutes the blue solution slightly, which would cause a shift toward shorter wavelengths, I don’t think that’s responsible for the majority of the effect here.
If direct energy transfer is going on, then some of the excited 7DE4MC molecules give energy to molecules of the other dye instead of emitting light. Those molecules do not have any particular effect on the output wavelength of the blue dye, because they are not participating in lasing. (If anything, because they are back in the ground state, they can absorb, so they would tend to “push” the output very slightly toward longer wavelengths.)
If, on the other hand, the excited blue dye molecules are emitting photons, which are then being absorbed by molecules of the other dye, then we could see a shift in the blue laser wavelength. If the absorption of the other dye is stronger near the long-wavelength end of the emission of the blue dye, we can expect to see the blue laser wavelength become shorter as we add more of the other dye, and in fact we appear to be seeing at least some of that here. The effect, however, is not pronounced, and there is a fair chance that we are actually seeing both direct transfer and reabsorption of photons by the second dye.
Here is an example that seems to be less aambiguous. To make these photos, I first mixed a quantity of 7-DE-4-MC in acetone. Then I divided it into two parts, one of which I poured into the cuvette. I added another BASF dye, Lumogen F Rot 305, to the other part. (Like Orange 240, Rot 305 has excellent fluorescence efficiency, but does not absorb well at 337; it lases on its own when pumped by the nitrogen laser, though not as well as Orange 240.)
The photo on the left shows the pattern, the cuvette, and the spectra, with a modest amount of Rot 305 added. If you look at the spectrum of the cuvette, you will see that the red emission shows up there quite a bit before there is any red lasing from the dye solution. (There appears to be a hint of red lasing in the diffuse part of the output spectrum, but I think that’s an artifact. Notice the fact that the same area that has some red in it in the spectrum [left side] is blue-green in the output pattern on the target [right side]. My suspicion is that the light was bright enough to overpower the filters in the camera’s sensor. This is supported by the color of the bright laser spot, which is blue-green in the spectrum and nearly white in the pattern; to the eye, they are both blue.)
The photo on the right shows spectra with various amounts of Rot 305 added to the solution. Because the Rot 305 solution contained the same amount of 7-DE-4-MC as what was already in the cuvette, there is no dilution, and thus no opportunity for a dilution effect.
The top spectrum shows 7-DE-4-MC alone; the rest show changes as I progressively added more Rot 305. I have drawn fine black lines through the centers of the patterns on the image, so you can see that the center of the blue output moves very slightly toward longer wavelengths with increasing red concentration. Unless the absorption of Rot 305 is very even across the entire emission spectrum of 7-DE-4-MC, this is fairly clear evidence that much of the transfer is by direct energy transfer, and only a little of it is by emission and reabsorption.
(I am looking for an example in which there is a strong reabsorption effect. When I find one, I will put a set of photos here.)
These effects have been studied and reported in much
greater detail and with far more accuracy in the
literature on dye lasers than I can provide here; if you
are interested, you should do a literature search. That
would be wiser than taking my word for how these things
work, as I could easily be incorrect about various parts
of this.
...Back to the project:
I tried three possible blue candidates, and ended up using 7-Hydroxy-4-Methyl-Coumarin as the blue dye in the set. This dye, which is clearly very closely related to 7-Diethylamino-4-Methyl-Coumarin, is often called 4-Methyl-Umbelliferone. I dissolved it in 95% Ethanol, with a few drops of strong ammonia added. (4-MU lases best in basic solution.)
[I will note here that I am glossing over quite a bit of effort. Trying to find compatible Red and Green emitters for the three potential blue candidates took about two days.]
Once I got a solution of 4-MU lasing nicely I added some Fluorescein to get green. Fluorescein absorbs only moderately well at 337.1 nm, and is not optimal for nitrogen-laser pumping on its own, but it tolerates basic solution extremely well and might be expected to take energy from 4-MU, as in fact it did. Too well, in fact: I had to dilute it considerably in order to get any blue lasing at all.
Once I had blue and green I tried Rhodamine B for Red; but adding even a very small amount to the mixture, far less than would have been required to get any lasing in the red, quenched the Fluorescein, and the green just went away.
Fortunately, some time ago a friend had donated a small amount of Rhodamine 640 Perchlorate, which turns out to be compatible with the other two dyes, and can lase in basic solutions.
The next issue was achieving the proper balance. It was only moderately difficult to get three colors at once, and I saw some very pretty output patterns —
— but they were not well balanced, and they did not produce a reasonably good beam.
In order to balance the three colors I was obliged to adjust the concentrations of all three dyes, which I did by either adding a tiny amount of one dye or another to the solution, by diluting the solution, or both. One problem is that if you get any of the dyes too concentrated, it tends to quench. If the amount of output decreases as you add more dye, that’s almost invariably a sign that you need to dilute the dye solution.
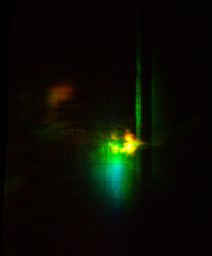
After a certain amount of madness, I finally managed to get close to a reasonable balance:
Here is a spectrum:If you compare this photo with the photo of 7-DE-4-MC lasing, you will notice that combining three dyes in one cuvette is not a good way to get lots of output. In fact, dividing the pump laser beam into three beams, using each of them to pump a cuvette containing one dye (or two dyes, where one does not absorb well at the pump wavelength, so the other is transferring energy to it), and then using optics to combine the three beams, is likely to get you considerably more RGB light. Still, it was a fun project, in addition to being instructive. When I began it I did not recall seeing simultaneous 3-color lasing reported in the literature on dye lasers (but see the citation below). I’m sure there are other sets of 3 or possibly 4 dyes that can be lased together with nitrogen pumping, and I would expect that even more could be done with an excimer laser as the pump source.
I will caution you, however: you are not likely to
achieve success if your nitrogen laser is at all wimpy;
mine appears to put out about 200 kW, and I suspect that
it is marginal for this application. In addition,
balancing the dye solution to get reasonably equal
output in all three colors is distressingly tweaky,
and you will have to exercise a great deal of patience
if you expect to attain success.
Reference
Dye-mixture laser tunable in three primary color regions
with a linear variable filter
Yasunori Saito, Koji Shimodaira, Akio Nomura, and Tetsuo Kano
Applied Optics, Vol. 34, Issue 3, pp. 432-434
To my [updated] mirror of the Joss Research site
To my current research homepage
the Joss Research Institute
Contact Information:
My email address is jon {a} jonsinger {d} org
My phone number is +1 240 604 4495.
Modified: Wed May 10 15:05:31 EDT 2017
Last modified: Tue Dec 21 02:52:31 EST 2021